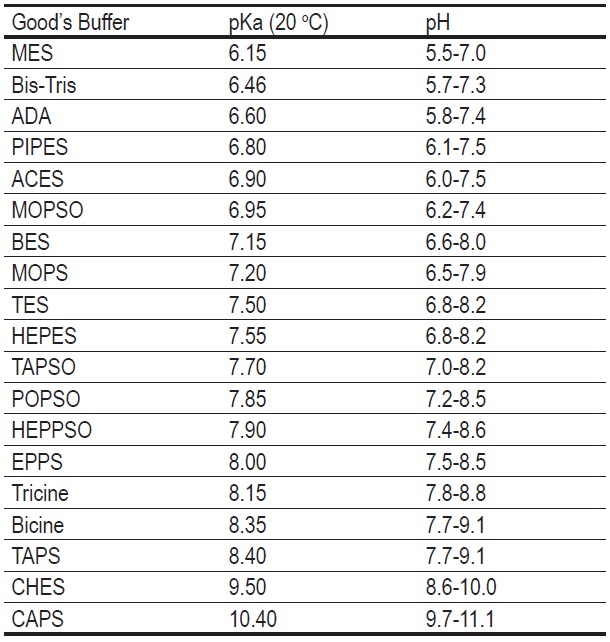
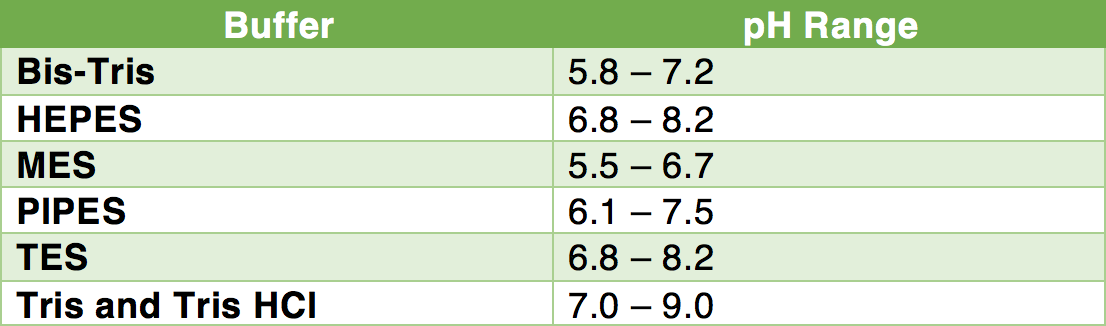
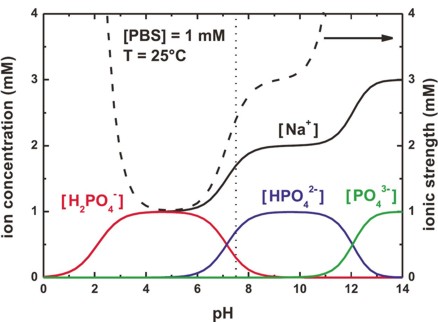
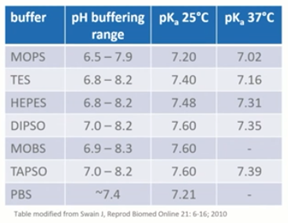
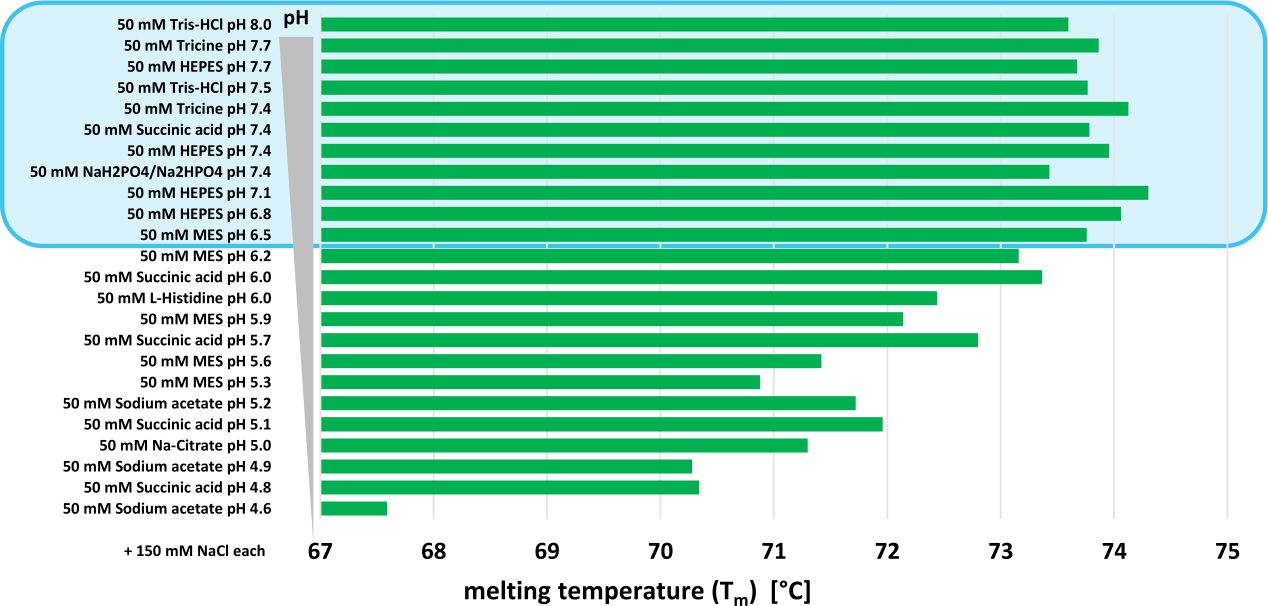
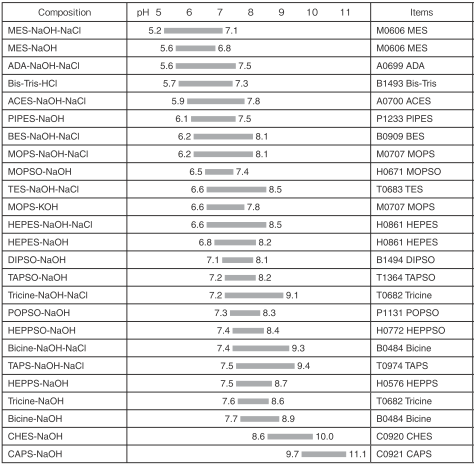
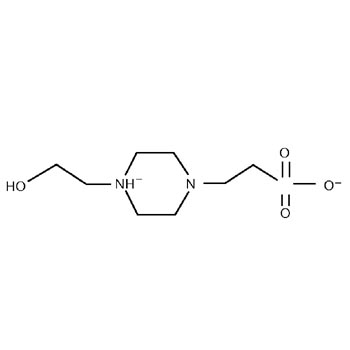
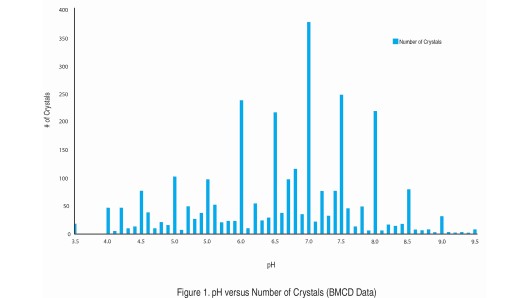
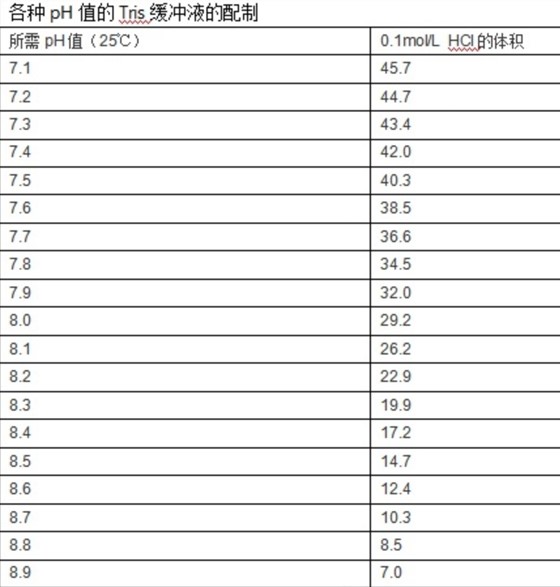
Quantitative cytotoxicity of buffers at various pH (MES buffer: pH 5.0,... | Download Scientific Diagram

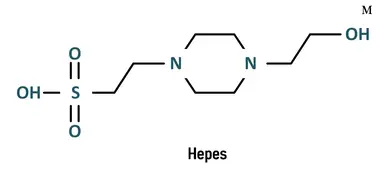
CAS 7365-45-9 The pH Value of The Hepes Buffer Range Is 7.2 to 7.4 - China Hepes Buffer Range, Hepes Buffer pH Value | Made-in-China.com

CAS 7365-45-9 The pH Value of The Hepes Buffer Range Is 7.2 to 7.4 - China Hepes Buffer Range, Hepes Buffer pH Value | Made-in-China.com

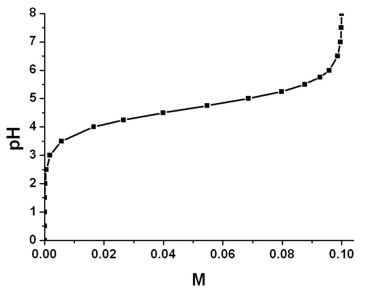
Titration curve of HEPES-sol, HEPES solution in HCl acidic medium by... | Download Scientific Diagram

Chemistry | Free Full-Text | Appropriate Buffers for Studying the Bioinorganic Chemistry of Silver(I)